

solutions

Crisis / Compliance Management
Managing the transition from a state of crisis to one of control rests on a capable, competent team.
In today’s fast paced environment where the strongest leaders are scattered across multiple important projects, anticipating and managing crises has become more difficult. Challenges may include an unexpected product safety or quality concern, compliance, organizational, or facilities/equipment issue.
We offer perspective, experience, leadership and support to complement your team in averting crisis, developing options and supporting execution in challenging times.

Transformational Change
More than ever before, the Pharma industry faces the growing need to accomplish more with less. Your need may be to manage more clinical studies, submit more dossiers, manufacture and test more batches, or revise more product labels, more quickly, with less staff. We have the depth of experience to understand your specific objectives and help design and deploy the correct organizational, operational, and technological solutions.

Interim Executives & Focused Support
Short term gaps in capacity and/or capability can hamstring an organization’s ability to work strategically and to meet operational requirements.
We listen, build relationships, and provide trusted experience to help you manage these gaps across Quality, Compliance, Manufacturing, Pharmacovigilance, Labeling, Program/Project Management, etc. We help prioritize benefits and risk, working efficiently and effectively to meet regulatory commitments and other imperatives (e.g. FDA Consent Decree, Warning Letter, 483, CAPA, etc.).
We take pride in structuring coordinated transition plans to help develop your staff and ensure sustainable continuous improvement. Please reference the sample Focused Support CAPA deck.
Product Lifecycle Management
Industry mergers, global marketing, and the need to differentiate products often mean product lines that, over time, have become far too broad. This negatively impacts both profitability and compliance.
Few organizations capture the advantages of understanding the relative cost/benefit and allocating the correct level of resources through lifecycle management.
We can help you refocus your organization on priority products by establishing criteria to screen your products/sku’s and implementing a product rationalization and out-licensing program.
company

Philosophy
We value lengthy partnerships with our clients based on understanding their needs and accomplishing transformational, sustainable gains in performance. While we recognize that our role is transitional, we strive for our impact to be long-lasting.
We are relentless in defining, communicating, and achieving a shared vision through teamwork.

Experience
Clients say our unique perspective with an average of 15 years of industry experience across major functional groups sets us apart. From R&D and Clinical Development to Regulatory, Quality, Manufacturing, and Sales & Marketing, we understand how the pieces fit together and help anticipate both opportunities and obstacles.
We set high standards for both designing and helping to implement change.

Methodology
Successful projects evolve from Initiation and Discovery through Design and Implementation. Leaders and staff are engaged in accomplishing clear goals and objectives across process, people, and technology. Success rests on a shared vision, structured teams, delegation, empowerment, regular communications, and accountability.
Established concepts and tools support successful project delivery (e.g., lean enterprise, organizational development, change management, web enablement, Six Sigma, process mapping, FMEA, 80/20, root cause analysis, ABC, statistical process control).

methodology
We Partner with our Clients, are Results Oriented, and use Transformational Project Management.
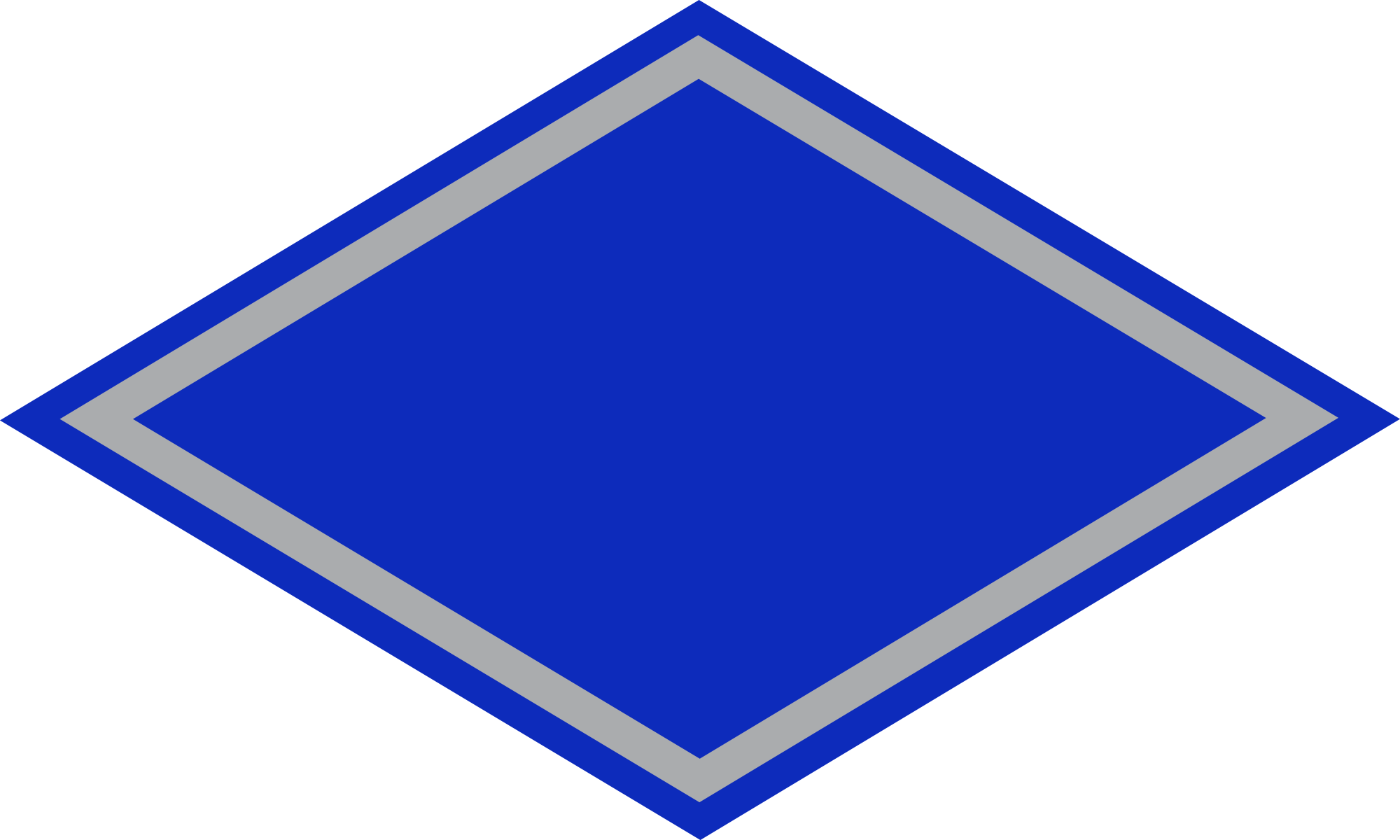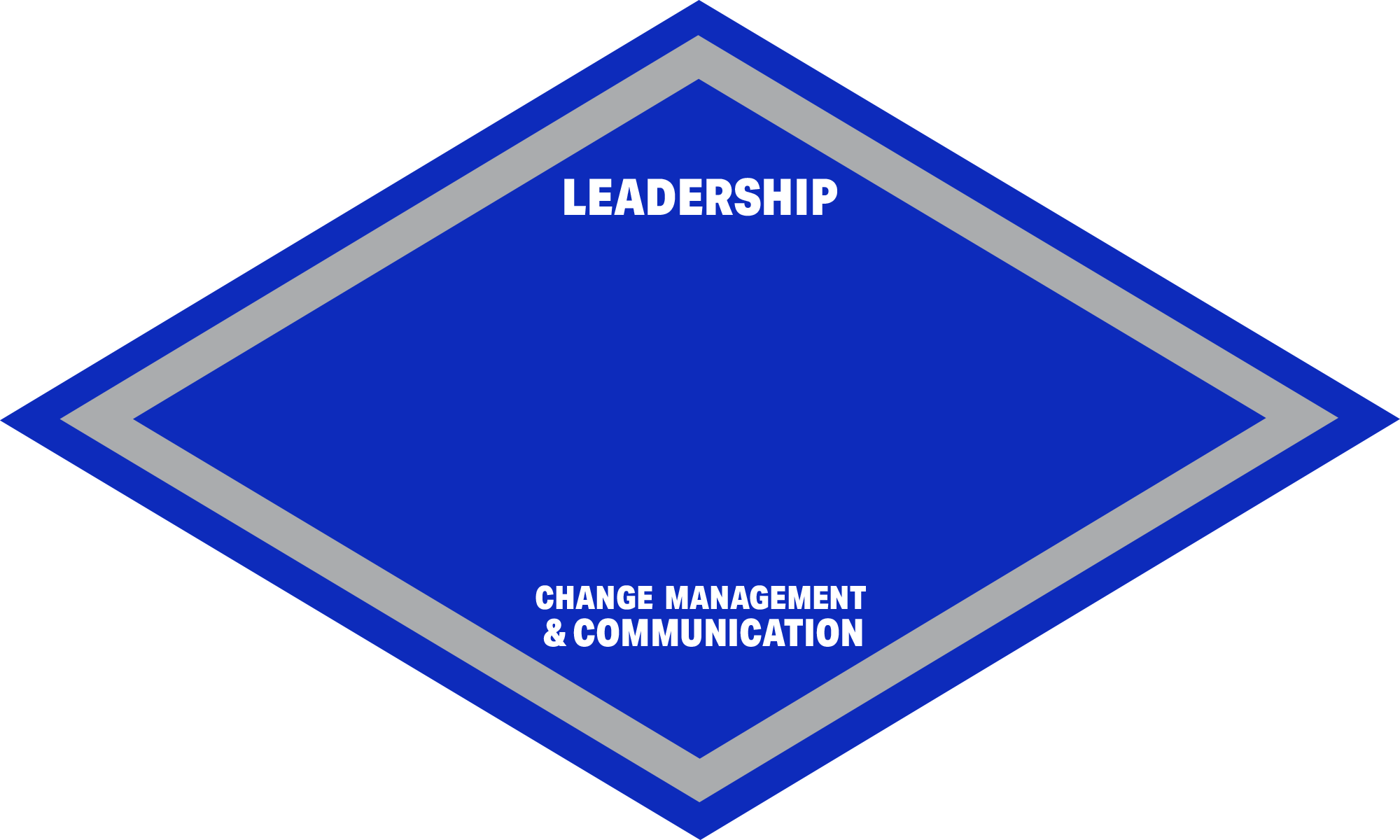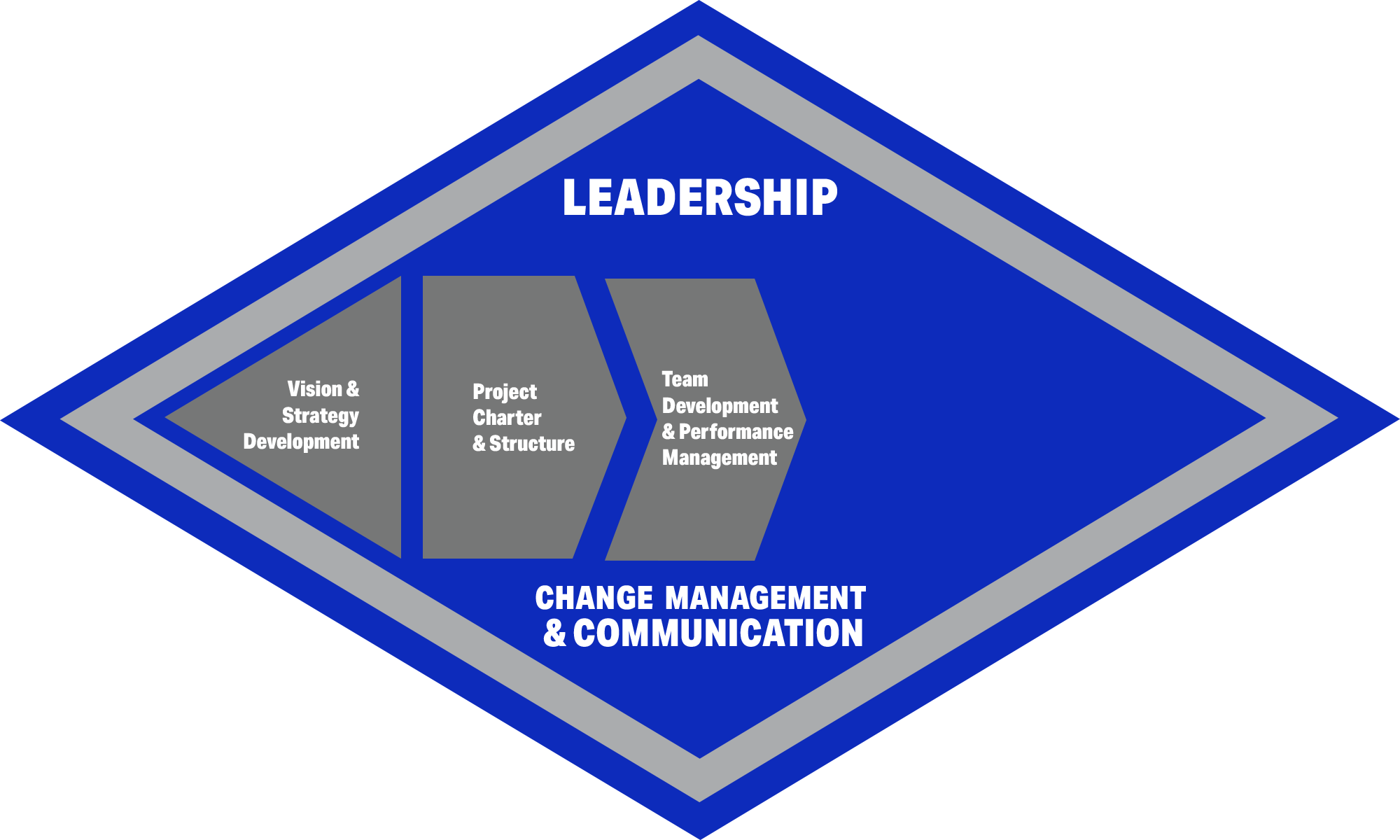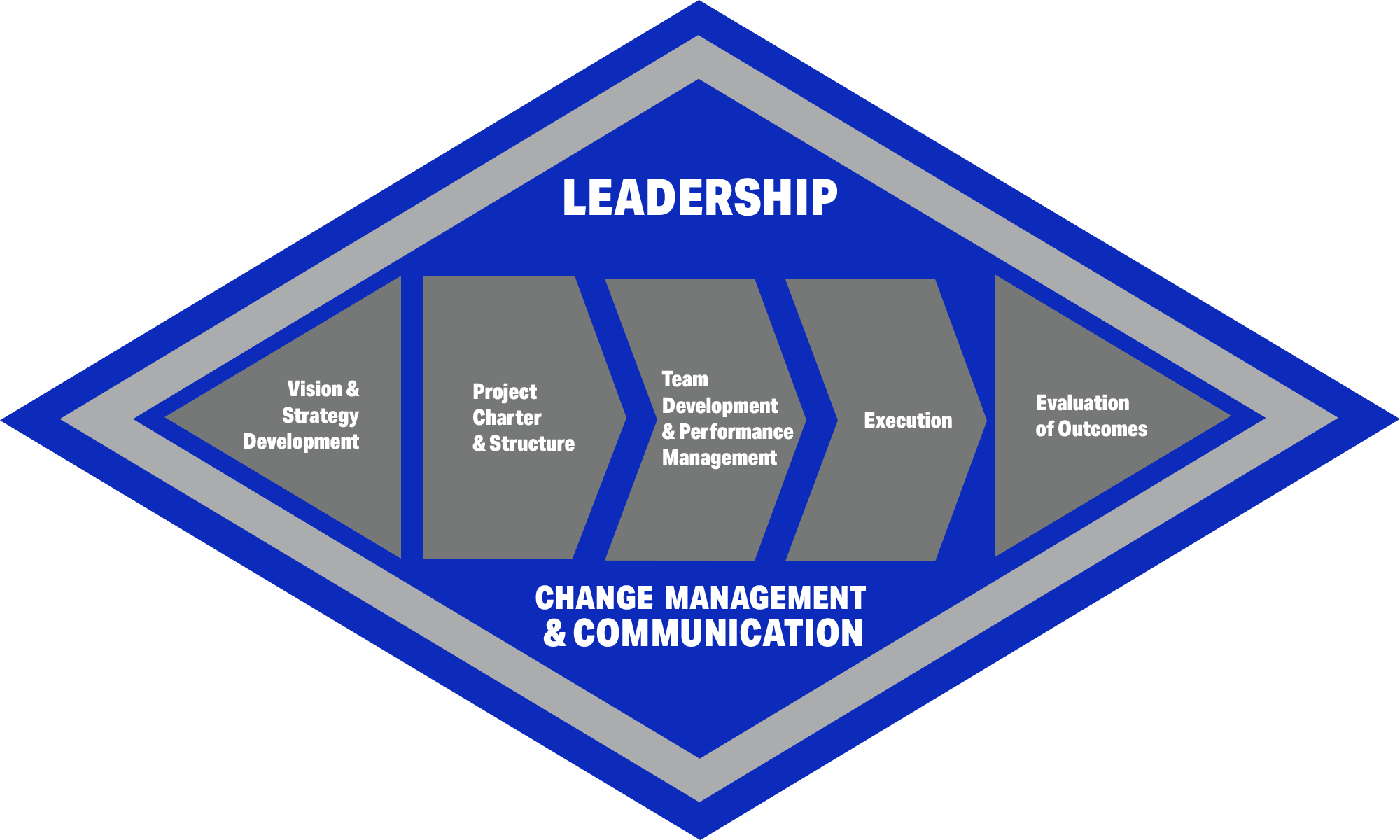
- We listen and build trusted relationships. We are experienced in taking the necessary time to understand your environment, culture, and vision.
- We partner with our clients using a structured, proven approach. Leaders and staff are engaged in defining strategy and accomplishing clear objectives across process, organization, and technology.
- Success rests on a shared vision, structured teams, delegation, empowerment, regular communications, and accountability.
- Established concepts and tools support successful project delivery, including:
- lean enterprise
- organizational development
- change management
- web enablement
- Six Sigma
- process mapping
- FMEA
- 80/20
- root cause analysis
- ABC
- statistical process control
- structured tech. evaluation (e.g. RFI/RFP)
We have partnered with a large number of life sciences companies to exceed expectations in achieving step-change improvements in performance.
clients & testimonials
Our consultants have worked closely with many of the largest and most respected life sciences organizations in the industry.




















What They Are Saying
“Splash Consulting has worked with us on several projects. Their experienced consultants are quick to identify the correct issues, and disciplined in defining and implementing sound strategies and project plans. They are effective in working with internal teams to achieve meaningful results and exceed expectations.”
– Senior Vice President, Pharmacovigilance (Top Ten Pharma Organization)
“Splash consultants are true visionaries and play a critical role in driving for results and getting the job done. They have integrated well with our staff to help design and implement optimal processes, organizations, and systems.”
– Vice President, Risk Management (Top Ten Pharma Organization)
“I have known the Principal of Splash Consulting for nearly 15 years and recommend his firm without hesitation. Splash provides an organized, strategic approach, and has the discipline, energy, experience, and diplomacy to work effectively with our team on aggressive timelines to achieve results.”
– CEO & President, Publicly Traded Biopharma Company
LET’S GET STARTED!
Please use the form below or call: 603.479.1068
Join Us: We are always looking to hear from outstanding people who share our philosophy, and have the depth of experience necessary to partner with our clients.
